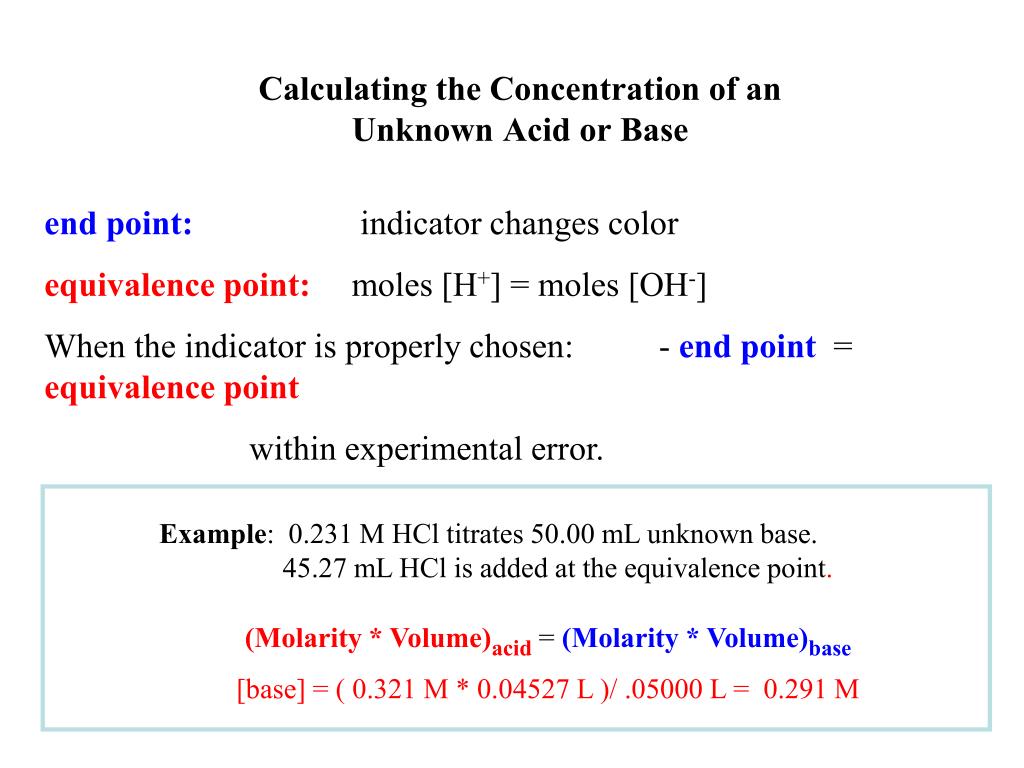
Using Titration To Find Concentration Of Unknown Acid . we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. suppose that a titration is performed and \(20.70 \: let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). \ce{naoh}\) is required to reach the end. Calculate the amount of sodium hydroxide in moles. The chemical nature of the species present in the unknown dictates which type of reaction is most. It is based on the neutralization. The moles of acid will equal. calculate the concentration of the hydrochloric acid solution. determining the concentration of an unknown solution using a titration.
from www.slideserve.com
suppose that a titration is performed and \(20.70 \: we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The chemical nature of the species present in the unknown dictates which type of reaction is most. It is based on the neutralization. calculate the concentration of the hydrochloric acid solution. The moles of acid will equal. determining the concentration of an unknown solution using a titration. Calculate the amount of sodium hydroxide in moles. \ce{naoh}\) is required to reach the end.
PPT AcidBase Titrations PowerPoint Presentation, free download ID
Using Titration To Find Concentration Of Unknown Acid determining the concentration of an unknown solution using a titration. Calculate the amount of sodium hydroxide in moles. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. The moles of acid will equal. \ce{naoh}\) is required to reach the end. The chemical nature of the species present in the unknown dictates which type of reaction is most. determining the concentration of an unknown solution using a titration. It is based on the neutralization. calculate the concentration of the hydrochloric acid solution. suppose that a titration is performed and \(20.70 \: let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh).
From www.chegg.com
Solved At the bottom it says " Enter the concentration of Using Titration To Find Concentration Of Unknown Acid calculate the concentration of the hydrochloric acid solution. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. The moles of acid will equal. It is based on the neutralization. Calculate the amount of sodium hydroxide in moles. determining the concentration of an unknown solution. Using Titration To Find Concentration Of Unknown Acid.
From learningschoolmurgkerny1.z13.web.core.windows.net
Chemistry Titration Questions And Answers Using Titration To Find Concentration Of Unknown Acid determining the concentration of an unknown solution using a titration. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The moles of acid will equal. calculate the concentration of the hydrochloric acid solution. It is based on the neutralization. suppose that a titration is performed. Using Titration To Find Concentration Of Unknown Acid.
From www.chegg.com
Solved CHEMISTRY • IDENTIFY WEAK ACID USING TITRATION Using Titration To Find Concentration Of Unknown Acid calculate the concentration of the hydrochloric acid solution. The chemical nature of the species present in the unknown dictates which type of reaction is most. The moles of acid will equal. suppose that a titration is performed and \(20.70 \: we will determine the concentration of the solution by titrating a known mass of a known acid. Using Titration To Find Concentration Of Unknown Acid.
From www.chegg.com
Solved Part II Titration of an acid of unknown concentration Using Titration To Find Concentration Of Unknown Acid determining the concentration of an unknown solution using a titration. Calculate the amount of sodium hydroxide in moles. It is based on the neutralization. The chemical nature of the species present in the unknown dictates which type of reaction is most. we will determine the concentration of the solution by titrating a known mass of a known acid. Using Titration To Find Concentration Of Unknown Acid.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Using Titration To Find Concentration Of Unknown Acid calculate the concentration of the hydrochloric acid solution. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. determining the concentration of an unknown solution using a titration. The chemical nature of the species present in the unknown dictates which type of reaction is most.. Using Titration To Find Concentration Of Unknown Acid.
From exoqbgfse.blob.core.windows.net
Sample Lab Report On Acid Base Titration at Michelle Hamilton blog Using Titration To Find Concentration Of Unknown Acid Calculate the amount of sodium hydroxide in moles. \ce{naoh}\) is required to reach the end. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The moles of acid will equal. calculate the concentration of the hydrochloric acid solution. suppose that a titration is performed and \(20.70. Using Titration To Find Concentration Of Unknown Acid.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Using Titration To Find Concentration Of Unknown Acid determining the concentration of an unknown solution using a titration. \ce{naoh}\) is required to reach the end. Calculate the amount of sodium hydroxide in moles. The chemical nature of the species present in the unknown dictates which type of reaction is most. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong. Using Titration To Find Concentration Of Unknown Acid.
From www.slideserve.com
PPT AcidBase Titrations A. Definition volumetric analysis of the Using Titration To Find Concentration Of Unknown Acid It is based on the neutralization. calculate the concentration of the hydrochloric acid solution. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate the amount of sodium hydroxide in moles. determining the concentration of an unknown solution using a titration. \ce{naoh}\) is required to reach. Using Titration To Find Concentration Of Unknown Acid.
From www.numerade.com
SOLVED EXPERIMENT 3ACID BASE TITRATIONS NEUTRALIZATION REACTION Aim Using Titration To Find Concentration Of Unknown Acid It is based on the neutralization. calculate the concentration of the hydrochloric acid solution. suppose that a titration is performed and \(20.70 \: The chemical nature of the species present in the unknown dictates which type of reaction is most. Calculate the amount of sodium hydroxide in moles. we will determine the concentration of the solution by. Using Titration To Find Concentration Of Unknown Acid.
From www.numerade.com
SOLVED Determine the concentration of the unknown acid using the above Using Titration To Find Concentration Of Unknown Acid let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). It is based on the neutralization. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. The chemical nature of the species present in the unknown. Using Titration To Find Concentration Of Unknown Acid.
From www.science-revision.co.uk
Titrations Using Titration To Find Concentration Of Unknown Acid Calculate the amount of sodium hydroxide in moles. determining the concentration of an unknown solution using a titration. The chemical nature of the species present in the unknown dictates which type of reaction is most. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. It. Using Titration To Find Concentration Of Unknown Acid.
From webstreaming.com.br
🎉 How to determine unknown amino acid from titration curve. Titration Using Titration To Find Concentration Of Unknown Acid The moles of acid will equal. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. determining the concentration of an unknown solution using a titration. It is based on the neutralization. calculate the concentration of the hydrochloric acid solution. The chemical nature of the. Using Titration To Find Concentration Of Unknown Acid.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Using Titration To Find Concentration Of Unknown Acid \ce{naoh}\) is required to reach the end. calculate the concentration of the hydrochloric acid solution. The moles of acid will equal. determining the concentration of an unknown solution using a titration. It is based on the neutralization. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh).. Using Titration To Find Concentration Of Unknown Acid.
From oneclass.com
OneClass Calculate the initial concentration of the unknown acid A by Using Titration To Find Concentration Of Unknown Acid suppose that a titration is performed and \(20.70 \: calculate the concentration of the hydrochloric acid solution. \ce{naoh}\) is required to reach the end. determining the concentration of an unknown solution using a titration. Calculate the amount of sodium hydroxide in moles. we will determine the concentration of the solution by titrating a known mass of. Using Titration To Find Concentration Of Unknown Acid.
From fity.club
Concentration Formula Using Titration To Find Concentration Of Unknown Acid determining the concentration of an unknown solution using a titration. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). Calculate the amount of sodium hydroxide in moles. The chemical nature. Using Titration To Find Concentration Of Unknown Acid.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Using Titration To Find Concentration Of Unknown Acid It is based on the neutralization. The moles of acid will equal. suppose that a titration is performed and \(20.70 \: determining the concentration of an unknown solution using a titration. we will determine the concentration of the solution by titrating a known mass of a known acid with your sodium hydroxide solution,. let's assume you. Using Titration To Find Concentration Of Unknown Acid.
From freeonlinehelpns.blogspot.com
Free Online Help QThe titration of 10.00 mL of a diprotic acid Using Titration To Find Concentration Of Unknown Acid let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). The chemical nature of the species present in the unknown dictates which type of reaction is most. \ce{naoh}\) is required to reach the end. Calculate the amount of sodium hydroxide in moles. determining the concentration of an unknown. Using Titration To Find Concentration Of Unknown Acid.
From www.slideshare.net
How do we find unknown concentrations of acids Using Titration To Find Concentration Of Unknown Acid calculate the concentration of the hydrochloric acid solution. let's assume you are titrating a strong acid (10 ml unknown concentration hcl) with a strong base (1.0 m naoh). determining the concentration of an unknown solution using a titration. It is based on the neutralization. Calculate the amount of sodium hydroxide in moles. we will determine the. Using Titration To Find Concentration Of Unknown Acid.